What is the Difference Between 0.45 and 0.22 Micron Filters?
There are different grade and pore size filters available in the market. But for sterility testing, only a 0.45-micron pore size filter is recommended in different pharmacopeias. The first question that comes to mind is that many bacteria are present in the environment which are smaller than 0.45-micron pore size like Brevundimonas diminuta then why don’t we use a 0.22-micron pore size filter which can retain these small size bacteria instead of 0.45 micron? Can bacteria smaller than 0.45-micron size pass through the filter paper of 0.45 micron if yes then why we are using 0.45 pore size? Can’t we use a 0.22-micron pore size filter for sterility testing? There are different questions that come to mind and create confusion.

When we perform sterility testing we use a 0.45-micron filter but when we prepare and filter the disinfectant solution we use a 0.22-micron filter. During batch preparation of SVP, LVP, and liquid injections we also use a 0.22-micron pore size filter for filtration of the batch. The reason behind these questions is the purpose of using these filters. Next, I will explain to you every aspect of this concept. First, let us understand the morphology of the filter paper with a particular pore size. Actually, membrane filters are not having single uniform-sized holes passing from top to bottom, but they are ramifications of channels throughout their whole thickness. So, because of the morphology of filters, it’s not possible for microorganisms to pass through the filter. Therefore a filter of 0.45-micron size can retain a large number of microbial cells smaller than the 0.45 micron.

In microbiology, we perform sterility testing to check any viable contamination in the product/sample by observing turbidity in the SCDM (Soyabean casein digest medium) or FTM (Fluid thioglycollate medium) media. If viable contamination is present in the sample then it could be retained on the filter paper during sample filtration and during incubation, we could detect that contamination. But what happens if we can’t detect the contamination which is present in the product or during sterility testing if we unintentionally destroy the contaminating microorganisms which were already present in the sample? It will lead to false negative results which mean contamination was present in the original sample but because of our testing problem or errors, we couldn’t detect the contamination in the product. That might cause the release of sterility failure batch in the market. Here in sterility testing our priority is to detect the contamination if present in the product. That is also mentioned in the pharmacopeia that the test must be carried out under aseptic conditions designed to avoid accidental contamination of the product during testing. For achieving these conditions, a grade A laminar airflow cabinet or an isolator is recommended. The test environment has to be adapted to the way in which the tests are performed. Precautions taken for this purpose should not adversely affect any microorganisms, which are to be revealed in the tests. The test is designed to reveal the presence of microorganisms in the samples used in the test. During sterility we apply a vacuum for filtration of the sample, if we use a 0.45-micron filter then microorganisms could be easily retained on the filter paper and the applied vacuum will not have an impact on the viability of the microorganism involved in the test but if we use 0.22-micron filter paper stringent pore size and applied vacuum leads to damage of microbial cell which will not further recover during the incubation time. That’s why we use a 0.45-micron pore size filter for sterility testing.
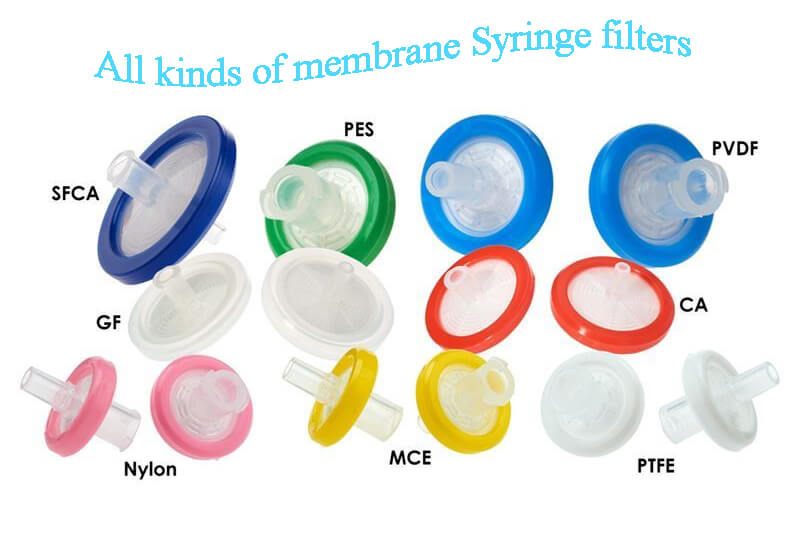
But during filtration of disinfectants or any liquid batch, we use a 0.22-micron pore size filter because in that our main purpose is to get a sterile solution by removing the contamination from the solution whether live or dead it doesn’t matter but the thing matters are that the solution must be sterile. Filtration is one of the methods of sterilization. So, by filtering through a 0.22-micron filter all forms of contamination could be removed. That’s why we use a 0.22-micron pore size filter for filtration.
Back to List
-
 下午4:09Weighing the Pros and Cons of PTFE/Silicone Septa
下午4:09Weighing the Pros and Cons of PTFE/Silicone Septa -
 下午4:05Decoding Vial Discard Guidelines: Ensuring Precision in Chromatography
下午4:05Decoding Vial Discard Guidelines: Ensuring Precision in Chromatography -
 下午5:01Navigating Micro Inserts for HPLC Vials: A Comprehensive Guide
下午5:01Navigating Micro Inserts for HPLC Vials: A Comprehensive Guide -
.jpg) 下午2:02Common faults and solutions of automatic samplers(2)
下午2:02Common faults and solutions of automatic samplers(2) -
 下午5:08Ensuring Sample Integrity: Navigating EPA Storage Vials Stability Guidelines
下午5:08Ensuring Sample Integrity: Navigating EPA Storage Vials Stability Guidelines

