What is HPLC(High-Performance Liquid Chromatography)?
HPLC is an abbreviation for High-Performance Liquid Chromatography. “Chromatography” is a technique for separation, “chromatogram” is the result of chromatography, and “chromatograph” is the instrument used to conduct chromatography.
Ⅰ. Overview of HPLC
High-performance liquid chromatography (HPLC) is a column chromatographic separation technology that uses a liquid under high pressure as the mobile phase and uses a high-efficiency stationary phase with extremely fine particles. High-performance liquid chromatography has wide applicability to samples and is not limited by the volatility and thermal stability of the analytical object, thus making up for the shortcomings of gas chromatography. Among the currently known organic compounds, about 20% can be analyzed by gas chromatography, while 80% need to be analyzed by high-performance liquid chromatography.
High-performance liquid chromatography and gas chromatography do not differ significantly in terms of basic theory, the major difference between them lies in the difference in the nature of the liquid and gas used as mobile phase.
Ⅱ. Principles of HPLC
1. HPLC Analysis Process
The solvent in the liquid storage bottle is sucked into the chromatographic system by the pump, and then output, after flow and pressure measurement, it is introduced into the injector. The analyte is injected by the injector and passes through the chromatographic column with the mobile phase. After being separated from the column, it enters the detector. The detection signal is collected and processed by the data processing equipment, and the chromatogram is recorded. Waste flows into the waste bottle.
The gradient controller can also be used for gradient elution when encountering complex mixture separation (with a relatively wide polarity range). This is similar to the temperature programming of gas chromatography. The difference is that gas chromatography changes the temperature, while HPLC changes the polarity of the mobile phase so that the components of the sample can be separated under optimal conditions.
2. HPLC Separation Process
Like other chromatographic processes, HPLC is also a continuous multiple-exchange process of solutes between the stationary phase and the mobile phase. It separates different solutes by means of the difference in the exclusion effect caused by the distribution coefficient, affinity, adsorption force or molecular size of the solute between the two phases.
In the beginning, the sample is added to the column head, assuming that the sample contains 3 components, A, B and C, which enter the chromatographic column together with the mobile phase and start to distribute between the stationary phase and the mobile phase. Component A with a small partition coefficient is not easily retained by the stationary phase and flows out of the chromatographic column earlier. Component C with a large distribution coefficient has a long residence time on the stationary phase and flows out of the chromatographic column later. Component B has a partition coefficient between A and C, and the second elutes out of the column. If a mixture containing multiple components enters the system, each component in the mixture will flow out of the chromatographic column according to their distribution coefficients between the two phases to achieve the purpose of separation.
The separation of different components in the chromatographic process depends first on whether there are differences in the distribution coefficient, adsorption capacity, affinity, etc. of each component between the two phases. This is a thermodynamic equilibrium problem and the first condition for separation.
Secondly, when different components move in the chromatographic column, the bands broaden with the length of the column, and the separation is related to the diffusion coefficient between the two phases, the particle size of the stationary phase, the packing of the column, and the flow rate of the mobile phase. Therefore, the final effect of separation is the comprehensive benefit of both thermodynamics and kinetics.
Ⅲ. Working Principle
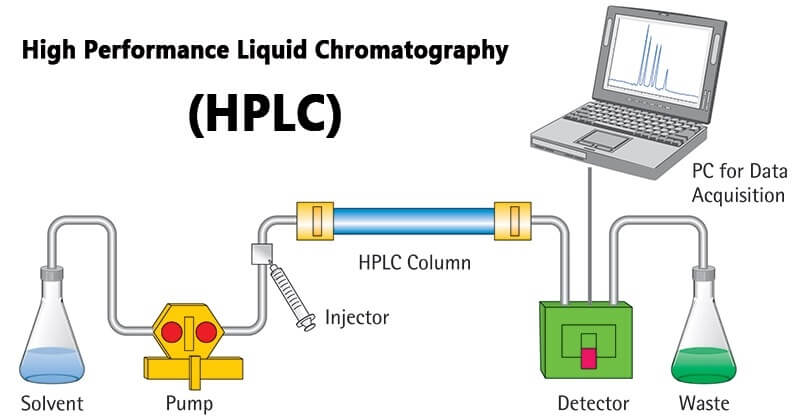
The mobile phase in the reservoir is pumped into the system by a high-pressure pump, the sample solution enters the mobile phase through the injector, and is loaded into the chromatographic column (stationary phase) by the mobile phase. Since each component in the sample solution has With different distribution coefficients, when the two phases are moving relative to each other, after repeated adsorption-desorption distribution processes, each component has a large difference in moving speed and is separated into individual components that flow out of the column in turn. , when passing through the detector, the concentration of the sample is converted into an electrical signal and sent to the recorder, and the data is printed out in the form of a graph.
Ⅳ. Characteristics and Advantages of HPLC
1. Characteristics
High pressure – the pressure can reach 150~300 Kg/cm2. The pressure reduction per meter of the chromatographic column is above 75 Kg/cm2.
High speed – the flow rate is 0.1~10.0 ml/min.
Efficient – up to 5000 trays per meter. Up to 100 components can be separated simultaneously in one column.
High sensitivity – the sensitivity of the ultraviolet detector can reach 0.01ng. At the same time, it consumes fewer samples.
2. Advantages
Fast speed—usually it takes 15-30 minutes to analyze a sample, and some samples can be completed within 5 minutes.
High resolution – the stationary phase and mobile phase can be selected to achieve the best separation effect.
High sensitivity – the ultraviolet detector can reach 0.01ng, and the fluorescence and electrochemical detector can reach 0.1pg.
Columns are reusable – different compounds can be separated with one column.
A small amount of sample, easy to recover – the sample will not be destroyed after passing through the chromatographic column, and a single component can be collected or prepared.
Ⅴ. Five Systems of HPLC
1. Sample Introduction Systems
The sampling system is divided into a manual sampling valve and an autosampler.
(1) Manual Sampling Valve
For the manual sampling valve, it is necessary to pay attention to cleaning the injection valve after each sample is injected during use to prevent the impact of residue on the analysis of the next sample.
It is also necessary to pay attention to the sampling method. Taking the 20μL quantitative loop as an example, we can choose to completely fill the quantitative tube or partially fill the quantitative tube. In order to ensure the repeatability of sampling, when choosing the method of fully filling the quantitative tube, it is necessary to inject more than 3 times the volume of the quantitative loop; when selecting the method of partially filling the quantitative tube, the injection volume should be less than half of the volume of the quantitative tube. That is, 1-10 μL.
(2) Autosampler
If an autosampler is used to inject samples, first of all, it is necessary to ensure that there are no air bubbles in the syringe, otherwise, the accuracy of the injection volume will be affected; secondly, there is enough sample in the sample vial to ensure that the sample needle can absorb the sample.
Regular cleaning of vials, caps, and septa is required to avoid cross-contamination. If the autosampler is not used for a long time, it should be noted that the corrosive mobile phase or washing solution (for example, alkaline or acidic buffer solution) must be completely replaced from the system. Also, to avoid bacterial growth, one vial should be filled with methanol and the injection should be repeated several times.
2. Infusion System
Infusion pumps are divided into constant pressure pumps and constant flow pumps according to the factor of constant output fluid. For liquid chromatographic analysis, the flow stability of the infusion pump is more important, because the change in flow rate will cause a change in the retention value of the solute, and the retention value is one of the main basis of chromatographic qualitative. Therefore, constant-flow pumps are more widely used.
Infusion pumps are divided into two categories: pneumatic pumps and mechanical pumps according to their working methods. There are screw-drive syringe pumps, single-piston reciprocating pumps, double-piston reciprocating pumps and reciprocating diaphragm pumps in mechanical pumps.
The high-pressure pump used by HPLC should meet the following conditions:
a. The flow rate is constant, without pulsation, and has a large adjustment range (generally 1 ~ 10ml/min);
b. Resistant to solvent corrosion;
c. Higher infusion pressure; for general separation, a pressure of 60×105Pa is sufficient; for high-efficiency separation, 150-300×105Pa is required.
(1) Structure and Principle of Reciprocating Plunger Pump
When the plunger is pushed into the cylinder, the one-way valve at the outlet (upper part) of the pump head opens, and at the same time, the one-way valve (lower part) where the mobile phase enters closes, and a small amount of fluid is output at this time.
Conversely, when the plunger is pulled outward, the one-way valve at the inlet of the mobile phase is opened, and the one-way valve at the outlet is closed at the same time, and a certain amount of mobile phase is sucked into the cylinder by its liquid reservoir.
The characteristic of this pump is that it is not affected by slight changes in the resistance of the rest of the entire chromatographic system, and continuously supplies a constant volume of the mobile phase.
(2) Pneumatic amplifying pump structure and principle
Principle: The low-pressure gas with pressure p1 pushes the large area (SA) piston A, and the small area (SB) piston B outputs liquid whose pressure increases to p2. The multiple of the pressure increase depends on the area ratio of the two pistons A and B. If the area ratio of A and B is 50:1, the gas with a pressure of 5×105Pa can obtain an output liquid with a pressure of 250×105Pa. This is a constant-pressure pump.
The pump head usually consists of two parts – a one-way valve and a sealing ring – a plunger rod. Check valves generally consist of a body, a plastic, or ceramic seat, and a ruby ball. Under the action of pressure, the jewel ball leaves the valve seat, and the mobile phase flows through the one-way valve; otherwise, under the action of the reverse force, the jewel ball returns to the valve seat, and the mobile phase no longer flows through the one-way valve.
Obviously, the fit between the jewel ball and the valve seat must be very suitable to prevent leakage of the mobile phase. In order to ensure that the check valve does not leak, two sets of jewel balls and valve seats are installed in some check valves, and there are also some check valves where the jewel ball is pressed on the valve seat with a suitable spring.
In different application fields, the material of the check valve body is different. For example, in the system considering biocompatibility, the valve body of the check valve is often made of metal titanium instead of stainless steel. In order to reduce costs and reduce maintenance costs, some manufacturers have also adopted replaceable ferrule-type plastic unit parts, and of course, their functions remain unchanged.
The infusion system refers to the high-pressure constant-flow pump, whose function is to provide a stable and accurate flow rate.
Solvent filter head: suction filter head, or sinker, has the main function is to filter the particulate impurities that may exist in the mobile phase. After long-term use, impurities may block the pores of the filter plate on the solvent filter head; or if the buffer solution is used for a long time, a film is likely to form on the surface of the filter head, which hinders the normal passage of the mobile phase.
In serious cases, even if it is a solvent that has been ultrasonicated, there will be bubbles in the PTFE infusion tube when the pump absorbs liquid, so the filter head should be cleaned frequently. The cleaning solvent can be ethanol or 30% dilute nitric acid solution, and the normal phase system should pay attention to the drying treatment of the filter head.
One-way valve: The function of the one-way valve is to ensure that the liquid flows in one direction, which is the guarantee for the stable infusion of the high-pressure constant-flow pump. During daily use, you can preliminarily judge whether the flow is normal by observing the pressure.
If the system has been equilibrating for some time, the pressure should be stable. But if the pressure fluctuates, it indicates unstable flow; if there is no pressure, it indicates no flow. These two situations are mostly caused by air bubbles or impurities mixed in the one-way valve.
If air bubbles are mixed in, the vent valve should be opened, and the air bubbles inside should be discharged by pressing the flush button. If the one-way valve is mixed with impurities, it must be cleaned. Ethanol can be selected as the cleaning solvent, pay attention to the direction of the marking ring when installing.
Sealing ring: The sealing ring is fixed on the plunger rod to prevent the liquid in the pump chamber from leaking, and is a key component to ensure the normal infusion of the pump head. However, the plunger rod and the plunger sealing ring will be worn for a long time, which is mainly related to the flow rate, operating pressure, and the mobile phase used.
When using a mobile phase containing salt, salt crystals may form due to dehydration or evaporation, and the salt crystals will cause wear of the seal ring and plunger rod when the pump is in motion. Therefore, it is necessary to rinse the sealing ring (between the plunger sealing ring and the secondary sealing ring) with pure water every day before and after the experiment, keep water in the cleaning tube to prevent the formation of crystals, and extend the life of the plunger rod and the sealing ring. service life.
In-line filter: In order to prevent impurity particles from the mobile phase from entering the chromatographic system, the pump is equipped with an in-line filter in the vent valve. The liquid flowing out of the pump outlet passes through the in-line filter, and the liquid flowing out through the exhaust pipe does not pass through the in-line filter.
After the instrument has been used for a certain period of time, it is recommended that the user clean the sintered stainless steel filter of the in-line filter. Remove the pressure cap with a wrench, take out the sealing ring and the sintered stainless steel filter for cleaning, and install them in place after cleaning. The cleaning solvent can be a 30% dilute nitric acid solution, and the normal phase system should pay attention to the drying treatment of the filter head.
3. Separation System
The separation system includes a chromatographic column, a guard column and a column thermostat.
Chromatographic column: The chromatographic column is the core of sample separation. Before using the chromatographic column, you must read the instructions carefully to understand the pH range, solvent tolerance range, pressure range and maintenance methods of the chromatographic column. After the chromatographic column is used, it is necessary to flush the chromatographic column in time. After flushing, the chromatographic column should be removed from the instrument, and the two ends should be sealed with the plugs provided by the manufacturer, and then stored in the chromatographic column box.
Guard column: The function of the guard column is mainly to prevent the contamination of the chromatographic column by highly adsorbed impurities, thereby prolonging the life of the chromatographic column. For different types of chromatographic columns, guard columns with corresponding fillers should be selected. It should be noted that the cartridge also has a life span and should be replaced regularly.
Column oven: Changes in column temperature may cause changes in retention time. To avoid this problem, the use of a column oven is recommended.
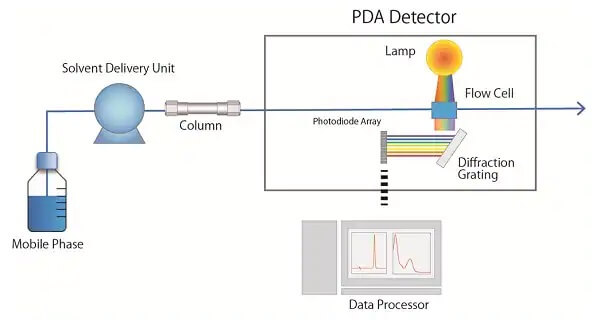
4. Detection System
There are three types of detectors commonly used in high-performance liquid chromatography: ultraviolet detectors, differential refractive index detectors, and fluorescence detectors.
(1) UV Detector
The detector is suitable for the detection of samples that absorb ultraviolet light (or visible light). It is characterized by a wide range of applications (such as proteins, nucleic acids, amino acids, nucleotides, polypeptides, hormones, etc.), high sensitivity (the lower limit of detection is 10-10g/ml), a wide linear range, and is resistant to temperature and flow rate changes. Insensitive, detectable gradient eluted samples.
(2) Differential Refractive Index Detector
Any sample component that has a different refractive index from the mobile phase can be detected using a differential refractive index detector. At present, this detection system is mostly used in the detection of carbohydrate compounds. This system has strong versatility and simple operation, but its sensitivity is low (the lower limit of detection is 10-7g/ml), and the change of mobile phase will cause the change of refractive index. Therefore, it is neither suitable for trace analysis nor suitable for the detection of gradient elution samples.
(3) Fluorescence Detector
For any substance with fluorescence, under certain conditions, the fluorescence intensity of the emitted light is proportional to the concentration of the substance. Therefore, this detector is only suitable for the determination of fluorescent organic compounds (such as polycyclic aromatic hydrocarbons, amino acids, amines, vitamins and some proteins, etc.), and its sensitivity is very high (the detection limit is 10-12 ~ 10-14g /ml), trace analysis and detection of gradient elution samples can be used.
A commonly used detector for HPLC is a UV detector. In daily use, the ultraviolet detector mainly needs to pay attention to the use and maintenance of the detection cell and deuterium lamp.
Detection pool: Long-term use of the detection pool may cause pollution. If the reference energy (REF ENERGY) of the instrument is normal and the measurement energy (SMPENERGY) is low, it may be caused by contamination of the detection pool. The detection pool can be cleaned.
To clean the parts of the detection cell, generally use about 1:4 nitric acid solution for ultrasonic cleaning, then clean with pure water and methanol solution respectively, then reassemble and push the detection cell body into the cell cavity, and tighten the screws of the cell plate. Note that during assembly, the cell glass and gaskets must be placed upright, so as not to crush the cell glass and cause leakage of the detection cell.
Deuterium lamp: The normal service life of the deuterium lamp can be more than 1500 hours. The service life of the lamp is related to the use time and turn-on frequency of the detector. Therefore, unnecessary start-up time should be saved as much as possible during use, and the switching frequency should be reduced to prolong the service life of the deuterium lamp.
If it is accurately judged that the deuterium lamp cannot be lit or the energy is too low, a new deuterium lamp needs to be replaced. When purchasing a deuterium lamp, you should consult and check whether the model of the deuterium lamp matches the model of the instrument. When changing to a deuterium lamp, refer to the relevant content in the instrument manual, and pay special attention to the position sequence of the deuterium lamp connecting wires.
5. Data Processing System
The system allows for the acquisition, storage, display, printing and processing of test data so that the separation, preparation or identification of samples can be carried out correctly.
Ⅵ. Applications
1. Environment: common inorganic anions and cations, PAHs, PCBs, nitro compounds, harmful heavy metals and their forms, herbicides, pesticides, and acid deposition components.
2. Agriculture: soil mineral composition, inorganic and organic components in fertilizers, feed additives, tea and other agricultural products.
3. Petroleum: composition of hydrocarbon families, trace components in petroleum.
4. Chemical: inorganic chemicals, synthetic polymer compounds, surfactants, detergent components, cosmetics, dyes.
5. Materials: liquid crystal materials, synthetic polymer materials.
6. Food: inorganic anions and cations, organic acids, amino acids, sugars, vitamins, fatty acids, flavors, sweeteners, preservatives, artificial colors, pathogenic microorganisms, mycotoxins, polynuclear aromatic hydrocarbons.
7. Biological: amino acids, peptides, proteins, ribonucleic acids, biogenic amines, polysaccharides, enzymes, and natural macromolecular compounds.
8. Pharmaceutical: human chemical components, various synthetic drug components, various natural plant and animal drug chemical components.
Back to List
-
 下午4:09Weighing the Pros and Cons of PTFE/Silicone Septa
下午4:09Weighing the Pros and Cons of PTFE/Silicone Septa -
 下午4:05Decoding Vial Discard Guidelines: Ensuring Precision in Chromatography
下午4:05Decoding Vial Discard Guidelines: Ensuring Precision in Chromatography -
 下午5:01Navigating Micro Inserts for HPLC Vials: A Comprehensive Guide
下午5:01Navigating Micro Inserts for HPLC Vials: A Comprehensive Guide -
.jpg) 下午2:02Common faults and solutions of automatic samplers(2)
下午2:02Common faults and solutions of automatic samplers(2) -
 下午5:08Ensuring Sample Integrity: Navigating EPA Storage Vials Stability Guidelines
下午5:08Ensuring Sample Integrity: Navigating EPA Storage Vials Stability Guidelines

