The Importance of the Cleanliness of the Headspace Vials
The cleanliness of the headspace bottle directly affects the analysis results.
The headspace vial is a container for instrumental analysis of the analyte, and its cleanliness directly affects the analysis result. Due to a large number of samples, a large number of sample vials need to be cleaned during the detection process, which not only wastes time, reduces work efficiency, but also sometimes causes deviations in the experimental results because the cleanliness of the cleaned sample vials does not meet the requirements.

Headspace vials are mostly glass and rarely plastic. Most laboratories wash and reuse vials. At present, the commonly used methods of cleaning the autosampler vial in the laboratory are mainly adding washing powder, detergent, organic solvent, and acid-base lotion, and then brushing with a customized small test tube. This conventional brushing method has many disadvantages, for example, the usage of detergent and water is large, the washing time is long, and it is easy to leave dead corners. If it is a plastic autosampler vial, it is easy to leave brush marks on the inner bottle wall. For glassware heavily contaminated with lipid and protein residues, it is recommended to use single-use headspace vials to avoid affecting the analytical results of new samples.

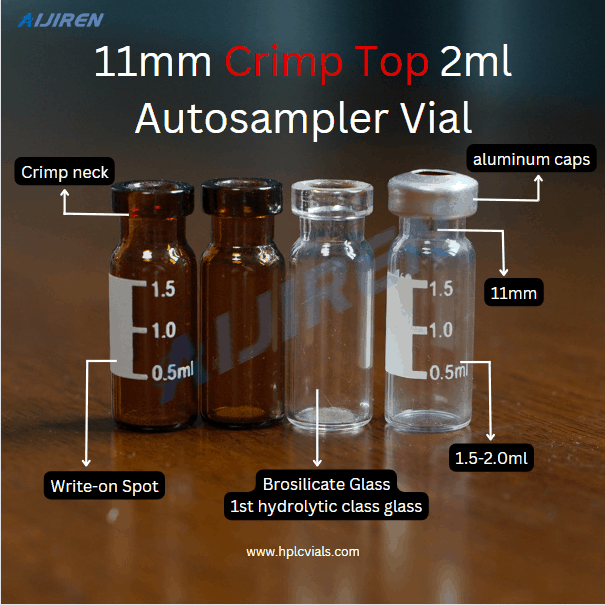
Most of the headspace vials are made of borosilicate glass, and its inertness can satisfy the analysis of most samples. The headspace bottle requires accurate volume, can withstand a certain pressure, and has good sealing performance and no adsorption to the sample.
Quantitative analysis involves accurate comparisons, which requires knowing the exact volume of the headspace vial. The simple method is to first weigh the empty bottle with a balance, then fill it with water and weigh it. Based on the density of the water at the weighing temperature, the exact volume of the headspace sample bottle can be calculated. For the same batch of headspace vials, the true volume of 5 of them can be accurately determined, and the average volume of the vials can be used as the true volume of the batch of headspace vials.
Headspace vials are available in a variety of volumes from 6 to 50 mL. It can be determined according to different situations, such as:
- According to the requirements of gas chromatography analysis.
- Depends on the sample condition. For liquid samples, headspace vials of about 10 mL are often used, because the analytical sensitivity depends on the concentration of the component to be measured in the headspace gas, not the sample volume. For solid samples, larger headspace vials should be used due to the bulk of the sample itself.
- Depends on column requirements. When using a packed column or a capillary column for split injection, the injection volume is generally 0.5~2mL, and a large-volume headspace vial is used. When the capillary column is used for splitless injection, the injection volume generally does not exceed 0.25mL.

Aijiren provides 6ml, 10ml, 20ml and other volume gas phase headspace vials. The crimp top headspace vial series strictly controls the bottle mouth to improve the tightness, and the precision-shaped neck improves the handling of the autosampler. ability. Standard GC and HPLC sample vials can be used for autosampler and sample storage. Flat-bottomed vials are suitable for moving downwards when the instrument is running. The flat-bottomed vials have a large contact area with the gasket and seal more tightly. The septum is made of PTFE composite silica gel to ensure excellent functionality and is more suitable for gas chromatographs to ensure that the septum will not fall into the sample vial during the injection process.
Back to List
-
 下午4:09Weighing the Pros and Cons of PTFE/Silicone Septa
下午4:09Weighing the Pros and Cons of PTFE/Silicone Septa -
 下午4:05Decoding Vial Discard Guidelines: Ensuring Precision in Chromatography
下午4:05Decoding Vial Discard Guidelines: Ensuring Precision in Chromatography -
 下午5:01Navigating Micro Inserts for HPLC Vials: A Comprehensive Guide
下午5:01Navigating Micro Inserts for HPLC Vials: A Comprehensive Guide -
.jpg) 下午2:02Common faults and solutions of automatic samplers(2)
下午2:02Common faults and solutions of automatic samplers(2) -
 下午5:08Ensuring Sample Integrity: Navigating EPA Storage Vials Stability Guidelines
下午5:08Ensuring Sample Integrity: Navigating EPA Storage Vials Stability Guidelines

